Abstract
Background:
The development of therapy-related MDS (t-MDS) is a rare but highly fatal complication of cancer therapies, with varying leukemogenicity associated with individual chemotherapeutic agents and radiation modalities. In recent years, an increase in t-MDS incidence explained by increased use of cancer treatment combined with an increasing number of cancer survivors has been reported. We investigated changes in t-MDS outcomes over time among cancer patients (pts) treated with chemotherapy and radiation using data from population-based cancer registries in the Surveillance, Epidemiology and End Results (SEER) Program of the US.
Methods:
A previously validated R package (SEERaBomb) was used to query all 18 SEER registries to identify adult pts (≥15 years of age) with multiple types of primary malignancies who were treated with chemotherapy and radiation as part of their initial cancer treatment, and subsequently developed MDS. Pts were observed from the date of first diagnosis of a primary malignancy until occurrence of MDS as second cancer, death, loss to follow-up, or end of the study period, whichever occurred earlier. Cases of MDS that developed at least 1 year (yr) following diagnosis and treatment of a primary malignancy were classified as t-MDS according to the World Health Organization classification. Primary cancers associated with at least 100 t-MDS cases were included in the analysis. Survival probabilities were compared for pts with: (1) primary cancers who did and did not subsequently develop MDS, (2) primary (de novo) MDS, and (3) t-MDS. Multivariate Cox regression analysis was performed to identify factors associated with overall survival (OS) in the t-MDS cohort, including the following covariates: age group [adolescents and young adults (AYA) (15-39 yrs); middle-aged (40-59 yrs); and older adults (≥60 yrs)], sex, year of diagnosis, race, and primary cancer type. Tests of significance were based on two-sided hypothesis at the .05 level.
Results:
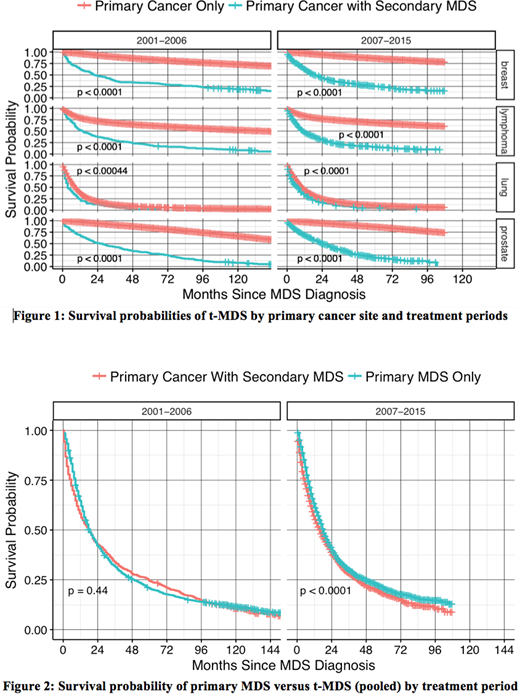
A total of 933,789 adult cancer pts diagnosed between the years 2001-2015 were assembled from all 18 SEER registries which included 926,552 primary cancers, 5,478 primary MDS cases, and 1,759 t-MDS cases [breast (528), lung (207), lymphoma (663) and prostate (361)]. Median survival of t-MDS in months (m) with (range) by primary site was as follows: breast: 17.5m (0.5-172.5); lymphoma: 10.5m (0.5-168.5); lung: 5.5m (0.5-88.5); prostate: 21.5m (0.5-170.5); and combined: 13.5m (0.5-172.5). Median latency periods in yrs with (range) to develop t-MDS were as follows: breast: 5 yrs (1-40); lymphoma: 5 yrs (1-38); lung: 2 yrs (1-21); prostate: 5 yrs (1-25); combined: 5 yrs (1-40). Figure 1 shows comparison of survival probabilities of primary malignancies with and without subsequent development of t-MDS over two treatment eras (2001-2006 and 2007-2015), organized by yr of diagnosis and primary cancer type. As expected, development of t-MDS was significantly associated with worse survival for all four primaries that contribute to the bulk of t-MDS cases. Figure 2 shows survival probabilities of primary (de novo) MDS versus t-MDS.
In multivariate analyses of factors associated with survival in the t-MDS cohort, the hazard ratio (HR) of death was significantly higher in middle aged adults [vs AYA, 2.20 (95% CI 1.34-3.62)] and older adults (vs AYA, 2.39 (95% CI 1.47-3.90)], in lung cancer [vs breast, 2.98 (95% CI 2.31-3.84)] and in lymphomas [vs breast, 1.467 (95% CI 1.18-1.83)], but was not influenced by sex [female vs male, 0.94 (95% CI 0.819-1.079)], race {[white vs black, 1.0 (95% CI 0.839-1.195)], [white vs other, 0.957 (95% CI 0.795-1.151)]}, or year of diagnosis of t-MDS [2001-2006 vs 2007-2015, 1.025 (95% CI 0.923-1.139)].
Conclusions:
In population-based analyses, the survival of t-MDS remains dismal with outcomes particularly worse with advancing age and following primary cancer sites that are typically managed with combined modality regimens including chemotherapy and radiation. Findings from the real-world population level data such as this should be used as outcome benchmarks in clinical trials to assess therapeutic effectiveness of experimental treatments.
Gerds:Apexx Oncology: Consultancy; Celgene: Consultancy; Incyte: Consultancy; CTI Biopharma: Consultancy. Nazha:MEI: Consultancy. Carraway:Jazz: Speakers Bureau; FibroGen: Consultancy; Balaxa: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Agios: Consultancy, Speakers Bureau. Maciejewski:Ra Pharmaceuticals, Inc: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis Pharmaceuticals: Consultancy; Ra Pharmaceuticals, Inc: Consultancy. Majhail:Incyte: Honoraria; Anthem, Inc.: Consultancy; Atara: Honoraria. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.